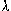 max (microns) = 2900 / T (K).
max (microns) = 2900 / T (K).PHYSICS 113
Assignment #2
SOLUTIONS
Chapter 4
1. What distinguishes one type of electromagnetic radiation from another? What are the main categories (or bands) of the electromagnetic spectrum?
ANSWER
(i) The "colours" of the electromagnetic spectrum can be distinguished by any one of the following quantitative variables: wavelength (); or frequency (); or energy (E). Once a number has been assigned to any one of these variables, the values of the other quantities can be calculated.
(ii) The main bands of the electromagnetic spectrum starting with the most energetic photons are:
(1) Gamma Rays
(2) X-Rays (Hard --> Soft)
(3) Ultraviolet
(4) Optical (Blue --> Red)
(5) Infrared
(6) Microwaves
(7) Radio Waves
Chapter 4
11. What type of electromagnetic radiation is best suited to observing a star with
a) a temperature of 5800 K?
b) a gas heated to a temperature of 1 million K?
c) a person on a dark night?
ANSWER
According to Wien's Law, the wavelength in microns at which a blackbody radiates its maximum
intensity is 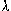 max (microns) = 2900 / T (K).
max (microns) = 2900 / T (K).
a) At T = 5800 K, 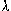 max = 0.5 microns. Since 1 micron (1
max = 0.5 microns. Since 1 micron (1  ) = 1 x 10-6 metres, the wavelength
expressed in metres is 0.5 x 10-6 m = 5 x 10-7 m. Using the spectrum chart from the class notes (or
the textbook), we conclude that this is the green part of the optical (visible) spectrum.
) = 1 x 10-6 metres, the wavelength
expressed in metres is 0.5 x 10-6 m = 5 x 10-7 m. Using the spectrum chart from the class notes (or
the textbook), we conclude that this is the green part of the optical (visible) spectrum.
b) At T = 1 x 106 K, 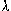 max = 0.0029 microns = 2.9 nm (1 nm = 1 x 10-9 m = 0.001 microns). This
corresponds to the far UV (or equivalently the "soft" X-ray) part of the EM spectrum.
max = 0.0029 microns = 2.9 nm (1 nm = 1 x 10-9 m = 0.001 microns). This
corresponds to the far UV (or equivalently the "soft" X-ray) part of the EM spectrum.
c) A person has a temperature of about 37 C. At T = 37 C = (37+273) K = 310 K, 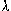 max = 9.4
microns = 0.0000094 m. This is the infrared part of the spectrum.
max = 9.4
microns = 0.0000094 m. This is the infrared part of the spectrum.
Chapter 4
12. Why is it dangerous to be exposed to x-rays but not (or at least as much) dangerous to be exposed to radio waves?
ANSWER
According to the formula, the energy (E) of a photon (particle of radiation) is proportional to the
frequency ( ) of the photon. Note that the frequency is inversely proportional to the wavelength
because c =
) of the photon. Note that the frequency is inversely proportional to the wavelength
because c = 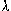
 and thus
and thus 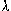 = c/
= c/ . Since X-rays are high (energy) frequency photons and radio
waves are composed of low (energy) frequency photons, X-rays are more energetic and can do
more harm to organic materials (such as cells). This is why gamma rays (highest energy) are used
to kill cancer cells. In fact, an X-ray photon has a frequency that is about a billion times
larger than that of a radio photon (thus an X-ray photon has a billion times more energy!!).
. Since X-rays are high (energy) frequency photons and radio
waves are composed of low (energy) frequency photons, X-rays are more energetic and can do
more harm to organic materials (such as cells). This is why gamma rays (highest energy) are used
to kill cancer cells. In fact, an X-ray photon has a frequency that is about a billion times
larger than that of a radio photon (thus an X-ray photon has a billion times more energy!!).